Clinically relevant antibodies in various Ig isotypes
Monoclonal antibodies (mAbs) have become a major tool in cancer therapy. They function through various mechanisms with the ultimate effect of priming either the innate or adaptive arm of the immune system to target tumor cells for destruction.
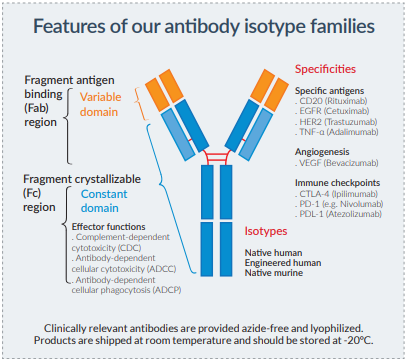
The efficacy of antibodies is governed by their bifunctional nature: the variable region confers antigen specificiy and the constant region triggers antibody-mediated effector functions by engaging a variety of Fc receptors.
These effector functions include complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).
One approach employed to enhance the efficacy of therapeutic antibodies is to modify the immunoglobulin (Ig) constant region.
Native and engineered isotypes
• Native isotype antibodies
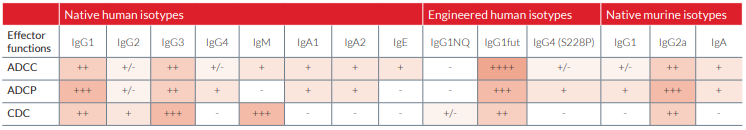
Physiological native isotypes trigger various combinations of effector functions that are summarized in the table below.
• IgG1NQ isotype antibodies
This isotype contains a N-glycosylation mutation of the constant region where potential asparagine (N) glycosylation sites are substituted by glutamine (Q) residues. These mAbs are non-glycosylated and their effector mechanisms mediated through the Fc receptor (FcγRI, FcγRII and FcγRIII), and the C1q component of the complement, are severely compromised or ablated.
• IgG1fut isotype antibodies
The constant region of these mAbs is not fucosylated. This results in dramatic enhancement of ADCC without any change in CDC.
• IgG4 (S228P) isotype antibodies
IgG4 antibodies undergo a process known as Fab arm exchange that potentially reduces their therapeutic efficacy. IgG4 (S228P) mAbs contain an engineered hinge region mutation (S228P) designed to prevent exchange of IgG4 molecules.